April 28, 2015
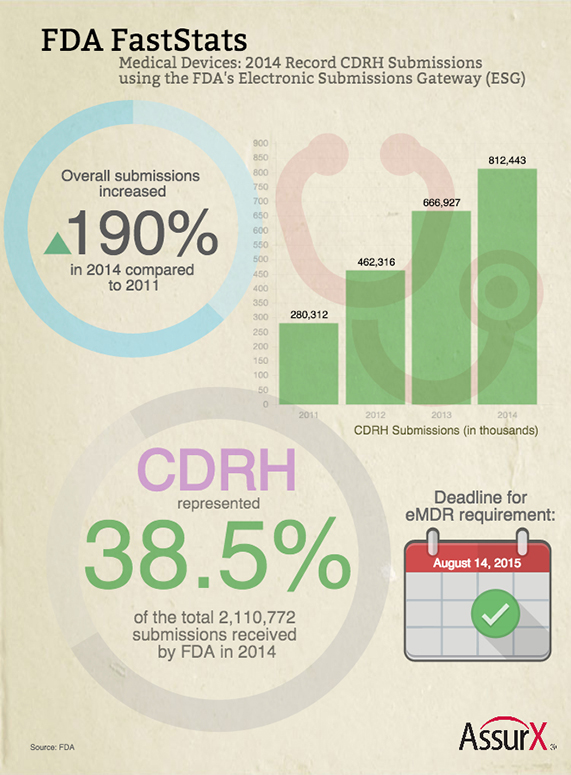
FDA FastStats: CDRH Shows Significant Growth in Electronic Submissions; Deadline Looming for eMDR – No more paper. That’s what the FDA requires from the medical device community starting August 14, 2015, with regards to electronic medical device reporting (eMDR). With the draft guidance initially introduced in 2009, and the final rule released in 2014, medical device manufacturers have a little over three months to comply. In the infographic shown below, CDRH submissions overall have dramatically increased through the years. Back in 2006, only 1,575 records were submitted electronically by CDRH to FDA. At year-end 2014, electronic submissions to CDRH had reached a record high of 812,443 and are expected to continue to rise going forward.
About AssurX:
With decades of expertise built into our quality management and regulatory compliance software platform, AssurX helps companies maintain quality and compliance, streamline workflow, control risks, and better manage your enterprise. Our configurable software and deep understanding of users’ needs produce a unique system that easily adapts as your business evolves.
AssurX is an ideal partner for regulated companies looking for better operational control and efficiency while meeting and exceeding compliance standards.
Other FDA FastStats Articles:
- FDA FastStats: CDRH Shows Significant Growth in Electronic Submissions; Deadline Looming for eMDR
- FDA FastStats: A Look Back at all FDA 483 Inspectional Observations for the Fiscal Year 2014
- FDA FastStats: A Look Back at 2013 Medical Device Warning Letters with Quality System Deficiencies
- FDA FastStats: A Look Back at 2013 Medical Device 483 Observations
- FDA FastStats: Got Safe Beef? New Report Suggests the US Doesn’t
Explore More Resources Now:
White Papers & Special Topics
Examine other technical and industry topics.
Videos
Learn all about AssurX and our products and hear directly from your peers.
Case Studies & Success Stories
Read detailed accounts of how customers in a variety of industries are using AssurX software.
Webcasts & Webinars
Access video replays of our most popular webcasts and webinars on a variety of industry-specific topics.
Brochures & Datasheets
Read about the AssurX platform as well as industry and software solutions.
Blog
Get the latest news and insights from AssurX and other industry leaders.