October 14, 2021
Solving problems that occur on the manufacturing floor is a lot like peeling an onion. In order to find the true root cause, you may have to peel back several layers during your investigation. Using the 5 Whys technique is such a popular root cause analysis tool, helping dig deeper to ensure corrective action goes beyond addressing just the symptoms.
This article discusses the basics of the 5 Whys technique. This includes when and how to use it, common mistakes and how a quality management system (QMS) can improve effectiveness.
What Is the 5 Whys Technique?
The 5 Whys technique involves asking why a problem occurred five times in succession. After each answer, you ask why again, digging into the next layer of the underlying cause. It’s important to note that five is just a guideline—you may need to ask even more times.
When to Use the 5 Whys
The 5 Whys is often the first root cause analysis tool teams choose when facing a quality issue. It’s good for simpler problems, or to help your team brainstorm possible root causes.
Unless a problem is very simple, the 5 Whys is often used alongside other root cause analysis tools like 8D or fault tree analysis (FTA). Cross-functional teams use multiple root cause analysis tools to look at the same event from different angles, much like looking at the facets of a gem.
Defining the 5 Whys Process
The process for using the 5 Whys can be broken down into the following steps:
- Create your team: Gathering multiple perspectives is critical to overcoming bias.
- Define the problem: To find the root cause, you first need an accurate problem statement.
- Ask why: Ask at least five times, possibly more.
- Evaluate possible causes: The answer to each why may have multiple potential subcauses, resulting in several possible root causes to evaluate.
Pitfalls to Avoid
The most common problem when using the 5 Whys is stopping too soon. People often try to decide on a solution before the root cause, not realizing that the issue they’ve identified is a symptom of the real root cause. Remember, there’s nothing to stop you from asking why 20 times if that’s what it takes to get to the true underlying cause. It’s also important to know when to stop, since going too far can unintentionally take you away from the problem.
In addition, another common mistake is not involving enough perspectives. Having diverse teams is helpful because everyone brings their own bias to the table. Sometimes, what first seems like a comment from left field doesn’t seem so unreasonable 20 minutes later. Furthermore, when doing an analysis, you might also be shocked at what someone from the manufacturing floor can tell you. Subject matter experts (SMEs) may understand a process, but they don’t live in it.
Documenting the Process: A Risk-Based Approach
A recent FDA and Medical Device Innovation Consortium (MDIC) report on improving corrective action offers a risk-based approach for when to go more in-depth with root cause analysis. In this framework, whether the incident is new or already has a documented solution drives the process. Risk is also a critical consideration, based on safety impacts and whether the product is still within your control.
For example, if it’s a first-time quality escape, you might use multiple tools, going all the way back to failure mode and effects analysis (FMEA). Conversely, if there’s already a 5 Why, fishbone diagram and FTA for a similar problem, you might use those results in a new 5 Why, adjusting for process changes.
In either situation, an enterprise quality management system (EQMS) becomes key to improving investigations, documenting root cause analysis, and creating corrective actions.
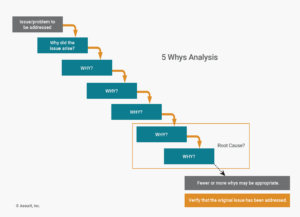
The objective of 5 Why is to keep probing until you are certain the root cause of a problem has been identified.
The 5 Whys: A Manufacturing Example
Let’s look at a simplified example of the 5 Whys in a manufacturing situation:
Problem statement: Machine is leaking oil
- Why is the machine leaking oil? >> Because there’s a gap at the edge of the filter gasket
- Why is there a gap at the edge of the edge of the gasket? >> Because it’s square rather than round like the other gaskets
- Why is the square gasket being used rather than a round one? >> Because we switched suppliers
- Why did we switch suppliers? >> Because they offered the gasket at a better cost
- Why do they offer it at a better cost? >> Because it’s thinner and cheaply made
The solution here might be to go back to your old supplier. However, you may want to dig down another layer to determine if your supplier qualification or procurement processes need to be updated.
Using the EQMS to Your Advantage
An EQMS equipped with 5 Whys for investigations is an advantage during root cause analysis. Not only does it help execute the process systematically, it also:
- Creates a searchable record for historical investigation and reference for resolving future issues
- Provides auditable documentation should regulators request more details on a particular issue
- Incorporates multiple levels of approvals for better collaboration, and documentation, dramatically improving efficiency and communication
- Allows you to link the outcome of a 5 Whys analysis to specific corrective action items, due dates, and responsible parties
Conclusion
The 5 Whys is a starting point for root cause analysis, and is often used with other tools. The process helps get to the root causes for further analysis. For best results, you’ll want to seek out multiple perspectives and be extra careful not to jump to conclusions.
Using the EQMS to centralize the process takes it to the next level with collaboration and reference documentation. The result of automating 5 Whys for root cause is improved efficiency, communication, and information gathering. Quality management system software provides a trusted data repository to support your continuous improvement strategies from premarket to postmarket processes.
RELATED READING:
Root Cause Analysis and CAPA: Creating a Closed-Loop Process
Human Error is a Precipitator for Root Cause Analysis, Not Blame



