March 31, 2022
As we move through 2022, we look forward to new opportunities, new challenges, and a return to normal. Unfortunately, one thing that won’t change is the likelihood of FDA CAPA and complaint observations, unless organizations act first. Recent trends show CAPA and complaint observations remain challenges for medical device organizations. CAPA and complaint Observations still top the FDA’s list for medical device manufacturers.
This article looks at these trends, and provides solutions to help decrease the likelihood of receiving CAPA and complaint observations while improving quality.
Data Trends
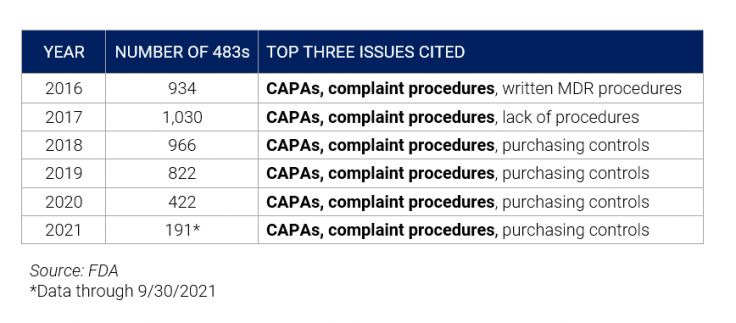
As far back as 2016, results consistently show inadequate procedures around CAPAs and compliant procedures. While purchasing controls are also of note, we’ll focus on the top two. The chart below shows FDA observations for medical device organizations over the last several years:
Common Issues with CAPA Observations
The CAPA, or corrective and preventive action, is the central process hub of any enterprise quality management system (#EQMS). It’s the process where the cause of all nonconformities and adverse events are documented. The importance is reflected in both the Code of Federal Regulations (21 CFR 820.100) and the ISO 13485 standard. However, the FDA continues to flag organizations for deficiencies in the management and resolution of CAPAs.
CAPA is an investigative process that solves problems, identifies root causes, takes necessary corrective action, and implements a preventive action. The intent of CAPA is to stop a problem or issue from happening again. There are many reasons the FDA has listed as the basis for deficiencies in CAPA management. Among the prevalent reasons are inadequate established procedures and/or late or incomplete investigations.
In many cases, insufficient CAPAs are due to outdated, siloed and manual quality systems. Lack of management support of a quality culture, and lack of emphasis on continuous quality improvement are key reasons cited. Therefore, making CAPA a top-down priority with the necessary commitment, resources, tools and training would reduce these observation trends.
How an EQMS can Reduce CAPA Observations
1. An EQMS Standardizes the CAPA Process.
Many organizations are still using spreadsheets to manage their CAPA process. This creates many challenges in terms of standardization. An integrated EQMS ties the entire enterprise into the CAPA system. As a result, all information resides on one system as electronic files.
A modern EQMS integrates processes to connect all core quality processes. For example, a complaint management solution or a supplier quality management solution can create CAPAs that share a common format.
2. An EQMS Centralizes CAPA Management.
Have one quality source of truth critical to increase both accuracy and efficiency. For example, utilizing a document management system within the EQMS eliminates the creation of multiple CAPAs for the same issue. Within a centralized quality management system, the CAPA process is streamlined to relate assignment, tracking, approvals, and related documentation.
3. An EQMS Automates the CAPA Process.
Utilizing a system that manages the status and various hand-offs is essential to the CAPA process, including staying ahead of critical due dates. EQMS software offers the necessary functionality to trace the status of one or many CAPAs, including current CAPA state, due date(s), and findings. Furthermore, the system can trigger “coming due” or “past due” alerts to keep the CAPA process moving.
Common Issues with Complaint Observations
21 CFR 820.198 mandates that a medical device company must maintain complaint files. This includes establishing and maintaining procedures for receiving, reviewing, and evaluating complaints by a formally designated unit. Unstructured, non-automated complaint management creates many gaps and management issues. For example, manual processes prevent complaints from being processed on a timely basis. Further, failure to properly classify, document and report adverse reactions could present significant risk to the brand, and a health risk to consumers.
In contrast to having no consistent procedures, a company may have what it considers to be an established complaint handling process. However, complaint observations commonly indicate that while there is a process, it does not pass muster for meeting standard FDA procedures. The procedures themselves may have gaps that allow for error or lack necessary details.
In many cases, manufacturers do not document clear procedures. However, a standard process is a requirement for conducting investigations, reporting (when necessary), and demonstrating strong process controls.
How an EQMS can Reduce Complaint Observations
1. Automation Improves Customer Service/Supplier Performance
An EQMS system for complaint handling can help you continuously improve your product and reduce the risk of complaint observations. Tracking complaints identifies common issues with low risk which can be solved quickly, as well as high-risk adverse events. Integration with supplier quality management allows you to directly address quality and compliance issues (which can connect into the appropriate CAPA investigations). Furthermore, capture findings from supplier audits to conduct root cause analysis.
2. Automation Enables Regulatory Reporting for Compliance
Automating complaint management streamlines the submission of required regulatory reports, such as EMDR for FDA or MIR to the EU. It also help you gain the functionality to help manage your customer complaints, identify trends, and submit the necessary adverse event reports in one system.
3. Automating Complaint Management Drives CAPA
As previously mentioned, an EQMS improves the CAPA management process through automation. A complaint management system that ties directly into CAPA can link multiple complaints to a CAPA, improving insights across the organization. A modern EQMS should enable a platform-based approach, allowing you to integrate processes as you’re read to build a robust quality system.
Conclusion
If the past is a good predictor of the future, warning letters citing deficiencies in CAPA and complaint management will continue. Fortunately, an electronic quality management system addresses fundamental issues with robust functionality. Focusing on quality improvements for CAPA and complaint handling process will minimize the likelihood of observations during FDA inspections.
Furthermore, when senior management adopts and prioritizes a robust CAPA management process, the emphasis shifts from responding to observations to preventing them. That support should include the implementation of an automated system since manual CAPA processes lack completeness in the investigations themselves. Fortunately, modern quality management technology can greatly improve speed and comprehensiveness.
Similarly, observations around customer complaints can be resolved with rigid processes with specific and clear instructions on how and who should address the various complaints, including clearly defining the timeliness of feedback to the FDA.
Therefore, taking the necessary steps of establishing robust procedures and processes, coupled with the support of senior leadership and the proper training of employees can go a long way in turning the trend of FDA CAPA and complaint observations.
Related Reading:
How to Simplify CAPA Management with a Cloud-Based QMS
Root Cause Analysis and CAPA: Creating a Closed-Loop System
About the Author
Kevin Tom is Director of Product Management for the Life Science and Manufacturing industries at AssurX. Kevin is responsible for driving customer value and product growth through innovation and strategic product vision. Kevin brings over 10 years of information technology experience in several functions, including ERP consulting, system implementations, and QA/project management for web-based configuration platforms. Additionally, he has 10 years of progressive product management experience in both the chemical and nylon plastic industries, and 10 years of supply chain leadership experience in both the generic drug and medical device markets.