November 16, 2021
In Stephen Covey’s 7 Habits of Highly Effective People, the author famously exhorts readers to “begin with the end in mind.” Quality leaders who are looking to get better results should consider what daily actions will help you achieve your goals.
In the continued spirit of World Quality Week which just passed (November 8-12, 2021), we’ve compiled six habits of highly effective quality leaders plus strategies for leveraging a QMS to foster operational excellence.
1. Quality Leaders Spend Time on the Manufacturing Floor
As far as daily habits of quality leaders go, spending time on the manufacturing floor is high on the list. In reality, quality isn’t about sitting in an office doing paperwork. It’s about what’s happening on the plant floor, even if many quality professionals are more comfortable doing their work from a desk.
In Covey’s words, “seek first to understand before being understood.”
To positively impact quality operations, quality leaders often:
- Visit the floor to see firsthand how manufacturing processes work
- Verify that processes are being followed
- Build trust with their by teams through demonstration of legitimate interest
- Interact one-on-one with front-line employees to understand their needs and concerns
2. Quality Leaders Use Technology Wisely
Today’s quality challenges are conducive with yesterday’s technology solutions. Wise use of technology is a must for quality leaders. Highly effective quality leaders focus on a well-structured, phased approach to automation and managing change.
Quality leaders watch emerging technologies like machine learning and artificial intelligence (AI) applications. However, they are strategic and realistic about making wise investments that drive real value today while keeping a finger on the pulse of developing solutions. Fortunately, many companies already have a wealth of QMS data they can use effectively with the right technology.
Examples include:
- Using supplier performance data to build supplier scorecards for benchmarking suppliers, creating custom inspection rules, and identifying emerging risks
- Analyzing document history data to identify unstable processes or those that need updating
- Creating trend reports from captured data, like complaint fields, to find patterns in terms and descriptions to identify key quality insights.
Focusing on building a foundation of QMS data provides long-term value and the data intelligence to drive continuous improvement.
3. Quality Leaders Adopt a Process Improvement Mindset
Focus on processes is a key part of being an effective quality leader. How well you’ve designed your processes will control your quality outcomes—not how many fires you’re able to put out in a day.
Consider, for example, how your department typically handles corrective actions. Are you constantly behind the curve, only dealing with overdue items? Or do you take a risk-based approach to prioritizing which corrective actions come first? Finally, do you have a closed-loop corrective and preventive action (CAPA) process that ensures problems are permanently solved?
Covey refers to this type of continuous improvement as “sharpening the saw.” Without taking time to grind down to fine detail and improve how you do things, quality will suffer.
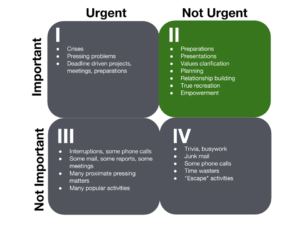
The Covey Leadership Quadrant illustrates a best-practice approach to managing business tasks in terms of urgency and importance. Quality leaders strive to spend the most time in Quadrant II. They are focused on proactive activities that improve processes, which in turn minimize the issues that end up in Quadrant I. Source: Covey Leadership Center, Inc.
4. Quality Leaders See Excellence Beyond Compliance
Quality management has traditionally placed great emphasis on compliance, and with good reason. Complying with regulations and standards is a basic requirement for ensuring product safety and quality. In medical devices alone, companies may need to comply with the EU Medical Device Regulation (MDR), Medical Device Single Audit Program (MDSAP), ISO 13485, and FDA’s 21 CFR 820.
With so many compliance obligations, it’s easy to get caught up in the details of requirements. Unfortunately, focusing on the end game without an eye on improving processes misses the point of quality management entirely. Furthermore, compliance alone will never be enough to achieve operational excellence.
To move beyond a “check box” mentality, manufacturers must leverage QMS tools that allow them to:
- Track and trend predictive quality metrics that show them where problems are most likely to happen
- Make risk management a central part of processes such as corrective action, supplier quality, and change control
- Conduct more effective root cause analysis to solve problems permanently
- Manage corrective actions more effectively and ensure compliance with risk-based reporting deadlines
5. Quality Leaders are Proactive
Getting caught up in daily fire fights is a risk to avoid. Effective quality leaders work diligently to stay ahead of issues with a proactive mindset, taking steps to prevent problems instead of just dealing with problems that have already occurred.
Take document management, for instance. Managing key documents in filing cabinets or even shared server drives causes costly and time-consuming errors. Consider, as an example, the impact of accidentally following an outdated specification. Or, losing an email related to a supplier corrective action request (SCAR).
Now compare these paper and spreadsheet approaches with tracking supplier documentation in an automated quality management system (QMS). There’s a reason why document management is often the first place manufacturers start with QMS automation. That’s because it helps you stay ahead of common problems that are easily avoided—so there’s less fire fighting needed overall.
The corollary in Covey’s 7 Habits: put first things first. After doing those things that are both critical and important, quality leaders should invest the lion’s share of their time on those tasks that are important but not urgent.
6. Quality Leaders Invest in Quality
Investing in quality is critical to continuous improvement. Investing in people, systems, and processes is what builds a long-term path to operational excellence, but the impact on your company can be far greater. Leaders make a solid business case for their quality investments with a keen eye on making better products faster with less risk and expense.
When the organization makes these investments behind a strong leadership team, they see visible proof that quality is a priority. As a result, employees are more likely to take ownership of quality as well. A commitment to quality from leaders creates a snowball effect that helps create and sustain a culture of quality across the enterprise.
An automated QMS is one of the most significant investments that a customer-centric organization can make. It provides an arsenal of tools to make employees’ jobs easier and eliminate problems at the source. At the highest level, a robust QMS supports a controlled approach to issue management that leads to operational excellence. It meets regulatory, compliance, and stakeholder needs while it delivers the greatest development impact.
Conclusion
To be effective, quality leaders must think strategically about their goals and the quality processes needed to achieve them. A “check the box” mentality is a crucial obstacle to overcome, and manufacturers must make operational excellence the goal rather than simply compliance. The future of quality lies in using technology wisely, adopting a proactive, process-based approach to accelerate improvement, and building the foundation of a culture of quality.
Additional Reading: EQMS Implementation: How to Lead Organizational Change


