January 3, 2023
Root cause analysis is a common weak spot in manufacturing, causing numerous quality issues, product safety problems, and enforcement actions. For example, recent headlines over baby formula safety indicate that it took months to conduct the investigation and alleviate formula shortages. In the food industry, the listeria pathogen in ice cream was notoriously difficult to root out. Identifying the true root cause of issues can be complex and time-consuming and requires knowing which root cause analysis tools to use in different situations.
This article examines three of the most useful root cause analysis tools and tips for when to use them.
The 5 Whys
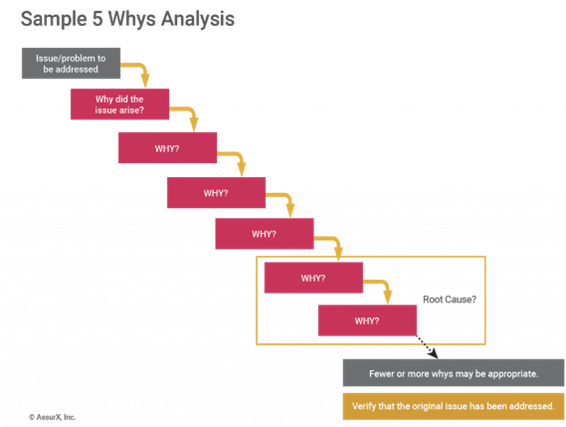
The 5 Whys is a method that asks why several times in succession to uncover deeper layers of a problem. This method is most useful when a team has trouble digging deep enough to get to the true root cause.
For instance, it’s very common for teams to ascribe problems to an employee training failure. However, this is a sure sign that they haven’t dug deep enough into the root cause. That’s because problems are rarely the result of human error. Instead, human error is usually just the symptom of the actual problem.
Let’s look at an example where an incorrect color cap was added to a bottle, and getting to the root cause with 5 Whys:
- Why #1: Why did the bottle have a white cap instead of a blue cap?
- The operator made a mistake.
- Why #2: Why did the operator make a mistake?
- They thought they needed the white cap.
- Why #3: Why didn’t they know it needed the blue cap?
- The standard operating procedure (SOP) wasn’t updated.
- Why #4: Why wasn’t the SOP updated?
- It wasn’t on the schedule for review.
- Why #5: Why wasn’t it on the schedule for review?
- Because there’s no SOP for how often it needs to be reviewed.
Note here that using the 5 Whys doesn’t limit you to asking why five times. More importantly, it’s about asking why enough times to peel back the onion to find the true root of the problem.
Failure Mode and Effects Analysis (FMEA)
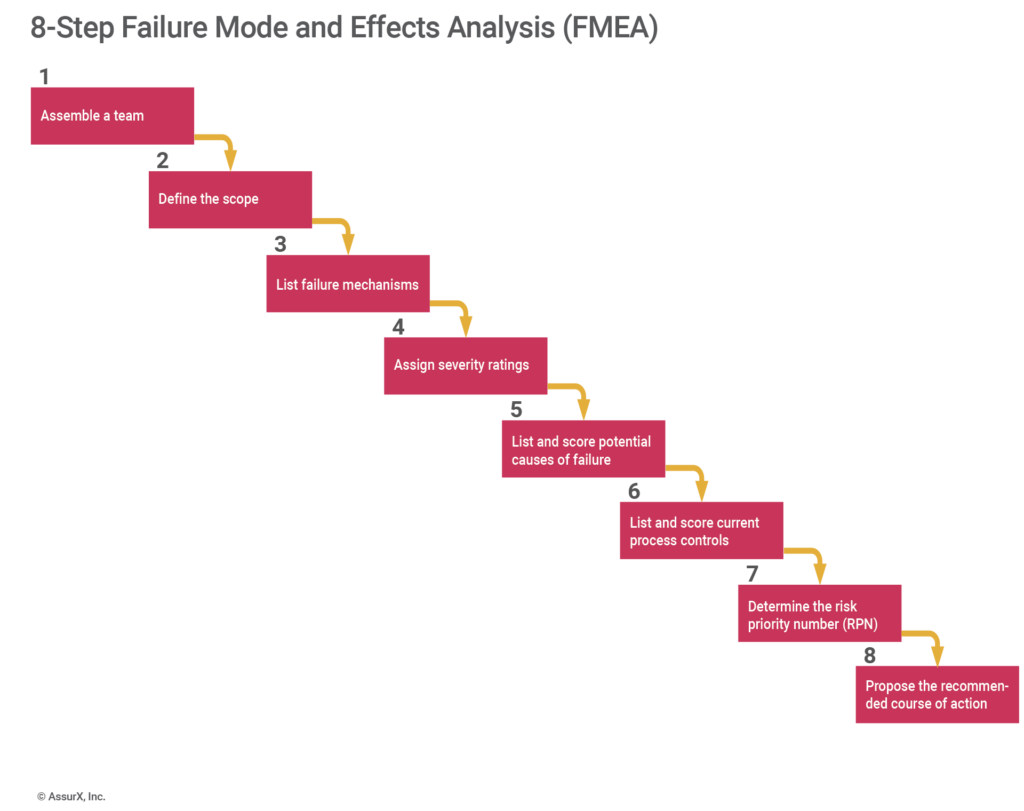
Failure mode and effects analysis (FMEA) is a tool that explores all possible ways a product could fail. FMEA also looks at the potential effects, helping prioritize risks based on severity, occurrence, and detection.
Companies should run an FMEA anytime they introduce a new process or product. While this is the most common use for an FMEA, it can also help with root cause analysis. In particular, an FMEA can be useful for nebulous issues you might not have seen before.
Let’s say you start receiving complaints about an issue with no idea where in the process it could have originated. An FMEA lets you map out the process to assess what could go wrong and where the issue might occur.
In situations where problems are not as obvious, it can help to see all potential problems laid out in black and white. Whether on a white board or spreadsheet, an FMEA helps visualize a process and get people thinking about potential root causes.
In the above example with the bottle caps, you might notice that step three is where the cap is added to the bottle. The solution might be to only have the correct color of bottle caps on the line, or adding a visual diagram for which one to use.
8D Problem-Solving
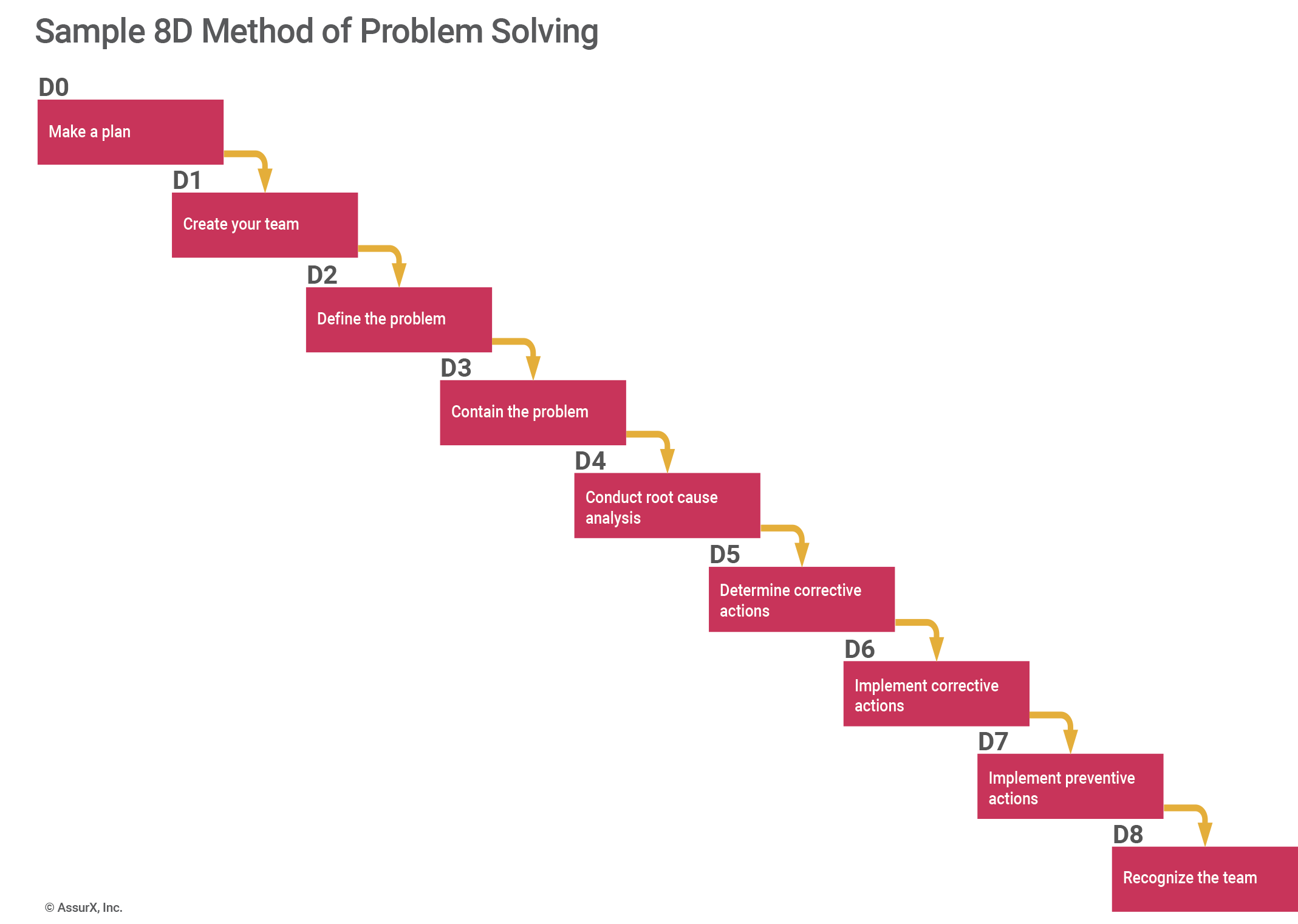
The 8D method is a structured, team-oriented problem-solving strategy best suited for investigating complex, recurring, or high-cost issues. The 8D method includes the following steps:
- D0: Make a Plan
- D1: Create your team
- D2: Define the problem
- D3: Contain the problem
- D4: Root cause analysis
- D5: Determine corrective actions
- D6: Implement corrective actions
- D7: Implement preventive actions
- D8: Recognize the team
Compared with earlier tools discussed here, the 8D method is more detailed and structured. This also makes it useful for when you need to document the problem-solving process for a customer. In many situations, these are the types of problems requiring a deeper technical dive into research, measurement and documentation.
One example would be if you’re having an issue with product labels falling apart, leading to a complaint. To solve the issue and reassure the customer that you’re taking appropriate steps to handle the issue, you:
- Make a plan for assessing the various stages of the manufacturing process (D0)
- Assemble a team from different areas such as quality, operations, and R&D (D1)
- Put together a description of the problem (D2)
- Describe what you’re doing in the interim to contain the problem such as sorting out products with warped labels (D3)
- Conduct a root cause analysis that looks at label measurements and why the nonconforming ones are out of tolerance (D4)
- Identify and implement the appropriate corrective actions (D5 and D6)
- Identify and implement preventive actions, including looking at other production lines or processes where the problem could occur (D7)
- Recognize your team for their contribution to solving the problem (D8)
The Role of the QMS in Root Cause Analysis
An enterprise quality management system (QMS) with built-in tools for 5 Whys, FMEA, and 8D can benefit root cause analysis in several ways. These include:
- Simplifying the process of gathering quality data and records related to your investigation
- Making root cause analysis records searchable to aid in future investigations
- Documenting your investigation in the event of a request from a customer or regulator
- Streamlining collaboration, reviews, and approvals for a more efficient process overall
- Allowing you to link investigation results to corrective actions, including helping track due dates and responsible parties
Conclusion
Root cause analysis is a fundamental technique for addressing quality problems and preventing them in the future. However, there’s no one-size-fits-all approach, creating issues where manufacturers have just one standard tool used in every situation. Whether it’s a problem blamed on operator error, one with unclear origins, or one requiring a deep technical dive, use the root cause analysis tools best suited for the situation.
An automated QMS can make root cause analysis tools such as 5 Whys, FMEA, and 8D more effective. Beyond helping standardize the process and document results, the QMS helps you see the problem within the context of other quality issues and investigations. The result is a more permanent problem resolution that minimizes risk and impact on the bottom line.
About the Author
Stephanie Ojeda is Director of Product Management for the Life Sciences industry at AssurX. Stephanie brings more than 15 years of leading quality assurance functions in a variety of industries, including pharmaceutical, biotech, medical device, food & beverage, and manufacturing.