January 21, 2021
In four months, the new EU Medical Device Regulation (MDR) goes into effect, which officially means it’s crunch time for devices that need to be certified to the new law.
What should companies be doing now to prepare?
The first steps for medical device compliance are understanding which devices need to be certified (or re-certified) by May 26, 2021. From there, companies need to get ready for certification audits by notified bodies (NBs), which includes adding new staff for non-EU companies, updating the QMS, and preparing documentation.
Let’s look at some of the details around these action items, and what’s next on the horizon in terms of MDR timelines.
Medical Device Compliance Transition Period
While May 26, 2021, marks the MDR compliance deadline, devices with a valid Medical Device Directive (MDD) certificate can be placed on the market until May 2024 (and placed into service until 2025).
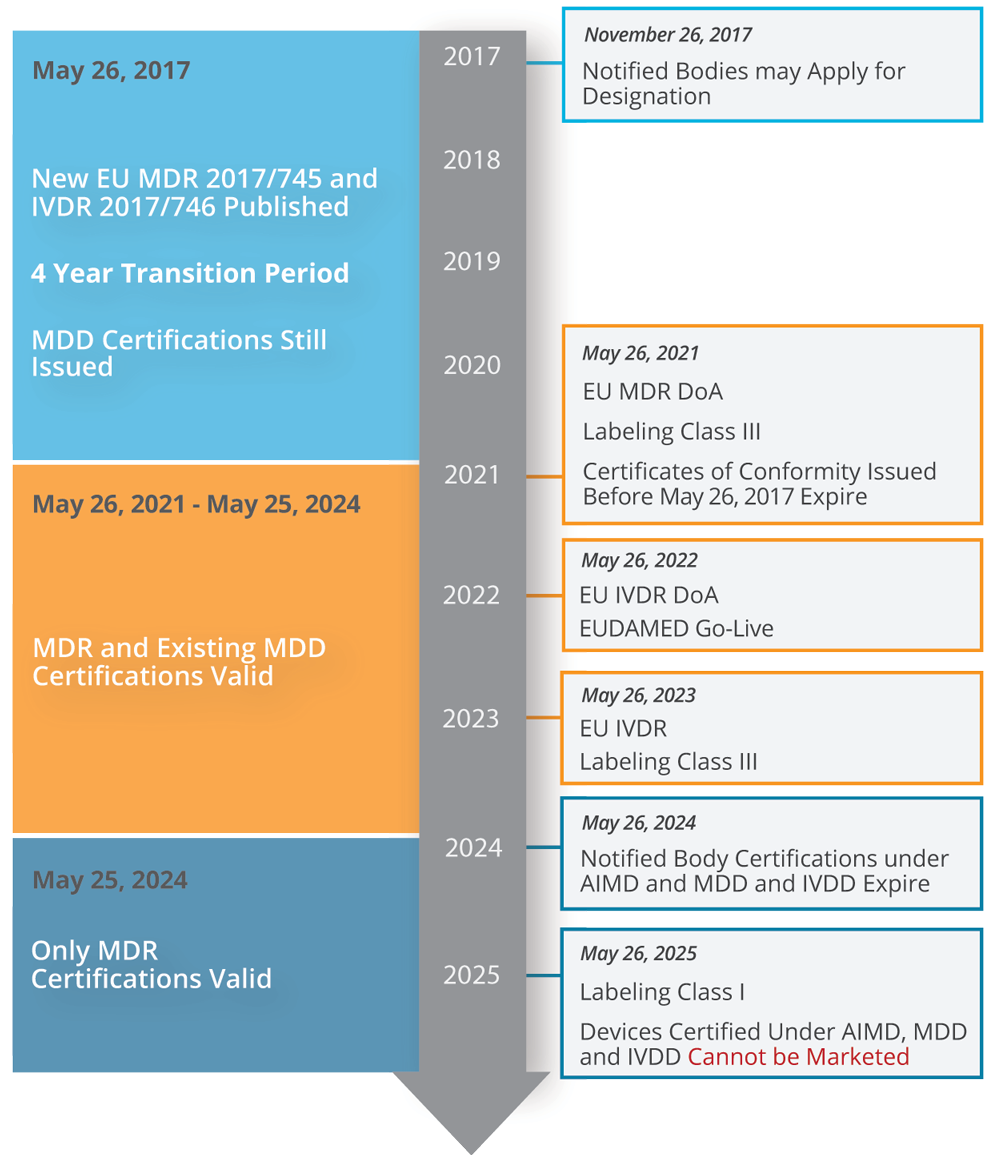
The original deadline for EU MDR was May 26, 2020, however, due to the COVID-19 crisis, the European Commission adopted a proposal to delay the date of application by a year and the EUDAMED ACTOR Module was released Dec 1, 2020, ahead of the May 2021 target.
However, certain conditions must be met:
- Devices must comply with MDR post-market surveillance, vigilance, and registration requirements.
- The MDD certificate must have been renewed by 2020.
- Class I (self-declared) devices are not eligible and must be certified to MDR 2017/745.
This means that many devices not previously regulated under EU law must undergo the MDR registration process. Even among those that don’t require certification now, complying with post-market vigilance and reporting requirements is a lot of work, considering the changes that went into the articles and annexes of the law.
Prioritizing Devices and Contracting with a Notified Body
The next step is conducting a product portfolio assessment to determine which devices need to be registered first and how you’ll prioritize them when working with your notified body (NB).
That means reviewing the new classification scheme for devices and identifying any exceptions that don’t need a new certificate until 2024 or 2025. Of those devices that need to be registered to MDR by May 2021, which ones will take priority?
In some situations, this may come down to a calculation of compliance costs versus device revenue. It’s important to consider that new medical device compliance will also demand more effort compared with devices already on the market.
Once you have your list, you’ll need to get in the queue with your NB as soon as possible. The time it took to get NBs certified to the new MDR has created a certification bottleneck, with many NBs only coming online in recent months.
Designating a Person Responsible for Regulatory Compliance (PRRC)
Under the new law, companies outside the EU need to establish a person responsible for regulatory compliance (PRRC) to act as a representative when dealing with regulatory authorities. This person must be separate from the authorized representative. While you can outsource both, each must have the required credentials and separation of duties without a conflict of interest.
Although the PRRC is a new requirement for marketing devices in the EU, the approach is similar to product registration in China and Japan, creating opportunities for harmonization.
Note also that authorized representatives now need to make checks to a host of additional areas. These include not only CE marks, labeling, and conformity assessments, but also post-market surveillance and recalls. Most controversially, they are also required to decide whether products comply with the regulations before either importing or refusing to import a product.
In the case of authorized representatives who are a subsidiary of a manufacturer, there is significant potential for conflicts of interest. It’s now the authorized representative’s responsibility to issue warnings to manufacturers and even report them to the authorities for non-compliance, which may make existing arrangements awkward.
Getting Technical Documentation and Data in Order
One element that sets apart the MDR from the previous MDD is the level of data and documentation required for compliance. The following months are a critical time for organizing that information, and the QMS can play an important medical device compliance role in preparing for the NB audit.
For example, struggling to find documents or being caught off-guard can draw additional questions elsewhere. Conversely, the ability to quickly access documents makes the audit faster and more efficient, while also making a better impression on the auditor.
An automated QMS offers a critical advantage, allowing companies to:
- Access records and compliance history from any quality process
- Ensure data integrity and data quality
- Provide verifiable audit trails required for MDR compliance
Updating the QMS for Medical Device Compliance
Companies should perform a gap analysis against Article 10 of the MDR, which outlines QMS requirements. Capabilities to focus on include:
- Change management
- Risk management
- Unique device identified (UDI) verification
- Corrective and preventive action (CAPA)
- Measurement and analysis
New technical file requirements also mean creating new document types and supporting workflows. An integrated QMS that ties these processes together can help companies with limited time and resources get more done faster, especially in the context of remote work arrangements.
Looking Ahead to EUDAMED and Beyond
The European database for medical devices (EUDAMED) is a central part of the new MDR legislation, pulling together market surveillance data for exchange between device manufacturers, competent authorities, and NBs.
The Actor Registration module—the first of six—went live in December 2020. Device manufacturers should be working with their NB now to complete this module. Reporting to the database is mandatory in May 2022 when all modules are live. The early release of the first module gives manufacturers time to register early, reduces the EUDAMED learning curve, and improves compliance with future releases.
Finally, medical device companies must monitor post-Brexit UK MDR requirements starting January 2021. While the Medicines and Healthcare products Regulatory Agency (MHRA) says it will consider international harmonization in new UK device legislation, current regulations are based on the previous MDD.
That means companies will need both a CE mark and a UK Conformity Assessment (UKCA) to sell devices in both the EU and the UK. MHRA requires manufacturers to register certain device types starting 2021, and CE markings will be recognized until June 2023.
The EU MDR deadline is coming up quickly, and the to-do list is long for manufacturers already coping with the pandemic and Brexit confusion. The key is to get organized now, leaning on the QMS to make the certification process for medical device compliance more efficient.
Resources:
- Regulatory Affairs Professionals Society (RAPS), MDCG clarifies remote audit expectations for notified bodies
- EU MDR, FAQ
- MDCG, MDCG 2020-4
- EU MDR, Article 10
- UK Government, Regulating medical devices in the UK


