October 12, 2022
Imagine you’re doing a pre-shipment inspection on multiple pallets containing product canisters. As a result of the inspection, you observe wrinkled labels throughout the lot. While the defect is cosmetic, the issue still requires quality incident management to correct it. Some of the labels may not be legible and the lots risk being sent back.
Upon further examination, you realize the lot is several weeks old. Unfortunately, that makes a root cause analysis investigation difficult, if not impossible. What once may have been a simple issue to correct has become time-consuming and costly.
Preventing issues such as the above all comes down to your quality incident management process.
This article explores five ways to improve root cause analysis (RCA) and quality incident management, recognizing the critical link between the two. We also examine how an enterprise quality management system (EQMS) helps integrate these processes to accelerate issue resolution.
1. Timely Reporting – Every Time
One of the top barriers to effective root cause analysis and quality incident management is the timeliness of reporting. As time passes and incident reports remain incomplete, it becomes more difficult to conduct an efficient and effective investigation.
The time to start quality incident management is at the time of initial occurrence. Fortunately, EQMS technology makes it far easier to record incidents faster. The ability to capture incidents in real time from any web-enabled device makes it possible to record them immediately. On the contrary, the likelihood of capturing accurate and important details surrounding the incident declines when documenting it on paper at the end of a shift.
2. Standardize the Definition of Incidents
Unfortunately, a common quality incident management issue occurs when there is no standard enterprise definition of incident types. In the canisters example above, operators might not report wrinkled labels as a defect or non-conformance.
One strategy for avoiding this problem is to conduct a failure mode and effects analysis (FMEA) exercise. An FMEA involves going through all the steps in your process to identify all the points where problems can occur. This, in turn, lets you drill down into the different types of incidents that you want employees to report.
Once you’ve done an FMEA, you must also close the loop by educating your team on what’s considered an incident. An automated quality management system allows you to link FMEA updates to new training requirements, ensuring production knows what to report. Unfortunately, many companies make the mistake of just filing away the FMEA and forgetting about it.
Standardizing your definition of incidents is essential from a QMS reporting standpoint. For instance, how large must a wrinkle be before it qualifies as a defect? Furthermore, where is the first point of inspection? Without consistency of definitions and responsibilities, the opportunities to analyze data for trends that can drive production efficiencies are lost.
3. Establish a Culture of Openness
Beyond aligning definitions for quality incident management, you must also create a culture where people aren’t afraid to report incidents. Unfortunately, people worry about blame for making an error. As a result, manufacturers have to normalize the capture of incidents to address this hesitancy.
Management must make it clear why reporting issues makes the organization better. A culture of collaboration where incidents aren’t viewed in a negative light is key to transparency and improving visibility. In addition, effective quality incident management through automation actually reduces the likelihood of future issues. Finally, while no one wants to take blame for certain issues, a QMS provides a clear record of control points and accountability. This eliminates the blame game, and allows quality management to focus directly on a clear point of failure.
In summary, help people understand that finding problems internally makes it easier to correct than after the customer finds them. This understanding is critical to being proactive, rather than being reactive and spending all your time fighting fires.
4. Capture Important Details for Quality Incident Management
Capturing enough detail to conduct a thorough investigation is another area where manufacturers struggle with quality incident management. Essential details to document include:
- When: Time and date of the incident
- What: Which product(s) were affected by the incident, and in what quantities
- Where: Which line the incident occurred on and the specific location on the line
- Who: Who observed the incident when it happened
Again, this is an area where an automated QMS makes it easier to capture more detail, so you can:
- Standardize the details you want documented
- Upload photos to quickly capture a detailed picture of the incident
- Launch a root cause analysis investigation and corrective action process directly from the incident
- Assign action items, responsible parties, and due dates to ensure you track and close the loop on the problem
- Report the issue promptly to regulatory authorities, where appropriate
5. Use the Right Tool for Root Cause Analysis
A number of root cause analysis tools exist to help manufacturers drill down into what caused an incident. However, these tools aren’t always put to use effectively for quality incident management.
Some of the most commonly used root cause analysis tools include:
- 5 Whys: The 5 Whys is useful for digging into deeper layers of a problem, asking why five or more times in succession to get to the root cause.
- Fishbone Diagram: A fishbone diagram can help brainstorm possible root causes to investigate. This ensures that you take into account the contributing factors of the 6Ms (man, machine, method, material, measurement, and Mother Nature), which you can then use in a 5 Whys analysis.
- Pareto chart: Based on the Pareto principle, Pareto Charts can help you identify and prioritize the most significant problems or contributors to a problem.
- FMEA: By evaluating your process and identifying areas where things could fail, an FMEA can provide a helpful starting point for investigating potential causes of a quality failure.
- 8D: The 8D problem-solving method is a detailed, comprehensive process that can be useful for addressing recurring problems. It’s also good for the investigation of high-risk problems that could have a serious impact.
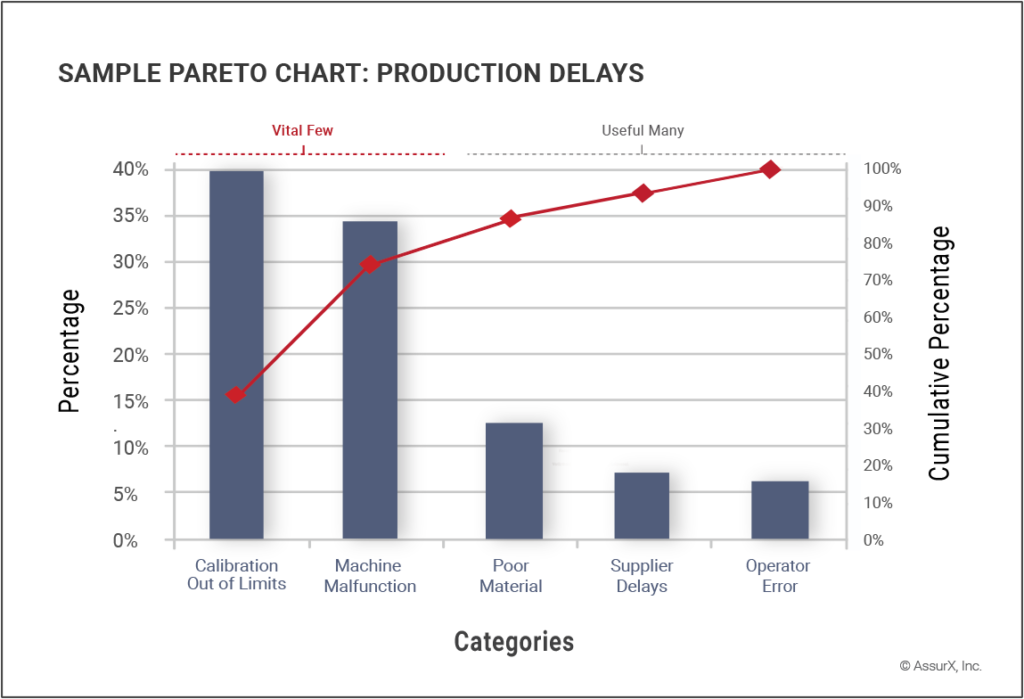
Sample Pareto Chart (also known as Pareto Analysis or Pareto Diagram). It is a visual tool used to analyze data sets that relate to a specific problem. When the Pareto Principle is applied (the 80/20 rule), the top 80% of measurable outputs represent the vital few problems based on severity and business impact. These problems are the most critical to resolve to have the most significant impact on quality assurance.
In the context of a QMS, it’s helpful to have an integrated system that walks you through each tool. This helps ensure you’re not missing anything, while allowing you to link the investigation to processes like corrective action, change control, and training management.
Conclusion
Effective root cause analysis and incident management are critical for improving quality performance. Getting these processes to work in sync requires standardization of what defines an incident and how employees must record them. Organizations must also strive to create a culture of openness that promotes reporting.
Root cause analysis and incident management tools in the QMS are essential to closing the loop on problems to prevent a recurrence. Companies that consistently use these tools are in a much better position to avoid recalls and reduce quality costs, while also improving product safety and customer satisfaction.
About the Author
Stephanie Ojeda is Director of Product Management for the Life Sciences industry at AssurX. Stephanie brings more than 15 years of leading quality assurance functions in a variety of industries, including pharmaceutical, biotech, medical device, food & beverage, and manufacturing.