October 21, 2021
Each day, quality leaders run across all types of problems. Fortunately, some can be corrected immediately. However, others need further investigation, such as a 5 Why analysis. Furthermore, there are often thornier problems: the ones that keep coming back, causing quality escapes and increased costs. In these situations, many manufacturers turn to the 8D method for root cause analysis.
8D stands for the Eight Disciplines, a problem-solving technique commonly found in automotive and other manufacturing industries. This article presents an overview of the 8D method, including the nine steps in 8D and how to get the most out of this technique.
What Is the 8D Method?
The Eight Disciplines or 8D is a problem-solving technique for addressing chronic quality problems. 8D is a team-oriented approach first put in use at Ford Motor Company in 1987.
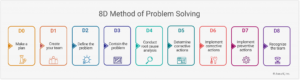
The 8D method covers the following steps:
- D0: Make a Plan
- D1: Create your team
- D2: Define the problem
- D3: Contain the problem
- D4: Root cause analysis
- D5: Determine corrective actions
- D6: Implement corrective actions
- D7: Implement preventive actions
- D8: Recognize the team
When to Use the 8D Method
While the 8D method is prevalent in the automotive industry, it’s well-suited and becoming commonplace for any company certified to ISO 9001 QMS standards. Compared with simpler tools like The 5 Whys, it is a more detailed and comprehensive process designed for solving recurring problems.
In the context of a risk-based approach to problem-solving and root cause analysis, 8D might be best for high-risk problems that your team hasn’t seen before. This contrasts with lower risk problems where there is a documented root cause analysis and corrective action is already in place.
Mistakes to Avoid When Using 8D
One shortcoming of the 8D approach is its focus on verifying that a corrective action was done—but not that the root cause was identified.
Unfortunately, you may see the same complaint recur. Most often, a recurrence indicates that corrective actions aren’t being verified and there is no evidence that they were effective.
The solution: implement 6-month or 8-month effectiveness checks after corrective actions. This ensures you monitor and confirm that failures are no longer occurring, and the team identified the real root cause(s).
8D’s focus on verifying implementation rather than effectiveness is a serious compliance gap for life science companies in particular. Not having adequate corrective and preventive action procedures is consistently a top violation identified in FDA inspections. CAPA violations represent the most common citation among Inspection Observations in 2020.
>> Learn how to create a closed-loop root cause analysis and corrective action process
The 8D Method in Action
Let’s say you have a problem where customers repeatedly complain about your products having an unpleasant odor upon opening. Because of the product quality and urgent nature of this new issue, you decide to do an 8D on the problem.
Here’s a simplified version of what that process might look like:
- D0: Plan: You create a plan to assess each stage of the manufacturing process to identify potential root causes.
- D1: Create your team: You bring together team members from multiple areas, including incoming inspection, quality, R&D, operations, and warehouse.
- D2: Define the problem: Here your problem statement might be that products have an unpleasant odor when opened.
- D3: Interim containment: Since you don’t know the root cause of the odor, initial containment might focus on adding a final inspection step before product leaves your plant.
- D4: Root cause analysis: During root cause analysis, EQMS data show a spike in complaints for products manufactured during the summer months. You use the information in a 5 Why, determining that a specific staging area gets sunny and overheated in the summer. This causes the packaging material to overheat and generate an odor. That material is included in the assembly of the final product in very small amounts.
- D5: Choose corrective actions: You change the layout of the warehouse floor to ensure products don’t overheat, adding an instrument to record temperature and humidity 24/7. In the long term, you build the case for buying environmental chambers to properly store the materials.
- D6: Implement corrective actions: Once you complete the change, you sample the products to ensure what leaves your facility doesn’t have a bad odor.
- D7: Preventive actions: You can look for other places where seasonal temperature changes could affect the stability of materials, products, or packaging.
- D8: Recognize the team: You add a note in your quarterly newsletter congratulating the team for successfully solving this difficult problem.
- To ensure you address the root cause, add an effectiveness check in six months to confirm that products no longer have the foul odor. An enterprise QMS will allow you to run an effectiveness check against related complaint categories. For example, you can check categories for “Odor,” “Location,” and others according to your categories.
8D in the EQMS
It’s essential to have an enterprise quality management system (EQMS) with built-in 8D and 5 Why tools. This gives you a pre-configured processes that you can start using immediately. It’s also important to have a configurable EQMS for automating existing quality processes with customized workflows, approval, and communication processes.
The EQMS supports your 8D process in a number of ways:
- Managing the team: The EQMS makes it easy to share documents, collaborate, and sign off on action items and plans. As opposed to sharing documents in email, information is centralized. Furthermore, automated reminders accelerate the process.
- Implementing corrective actions: You can use the EQMS to verify that a corrective action was completed, and add additional effectiveness checks to make sure the problem doesn’t reoccur. This is key to having a closed-loop corrective action process.
- Providing data for decision-making: Using EQMS data to evaluate root causes is critical to making consistent decisions. Having this real-world data means it’s not just the loudest voice in the room controlling the conversation.
Conclusion
8D is a systematic approach to fixing problems. It helps create short-term and long-term fixes that reduce impacts to products, customers, and business processes. As a best practice, execute effectiveness checks to identify the true root cause. An EQMS is critical for enabling success with the 8D technique. Automating workflows, tasks and timelines closes the loop on the 8D process for more effective, efficient problem-solving.