August 18, 2021
Data buried in file cabinets. Forgotten emails. Poorly documented processes. Unfortunately, these practices are not uncommon in food and beverage companies. As a result, poor food and beverage quality management leads to complaints, recalls, and regulatory action.
Of the top U.S. Food and Drug Administration (FDA) 483 inspection citations issued in 2020 to food and beverage companies, 874 citations were handed out for lack of documentation alone. From this perspective, an automated quality system based on digital data is a lot like investing in insurance. You don’t plan for a quality disaster. However, if one occurs, you’ve taken steps to protect your company, ensuring traceability and a quick response to problems.
In the modern world of automation, the food and beverage industry needs to sustain business compliance, reduce risks, improve quality, and improve customer satisfaction. Therefore, investing in an enterprise quality system (EQMS) is no longer an option—it’s a necessity.
This article post explores best practices for automating food and beverage quality management. We examine how to choose the right EQMS, where to start automating your processes, and the must-have ingredients for compliance.
Select a Configurable EQMS for Food and Beverage Quality Management
As you evaluate technology platforms for food and beverage quality and compliance management, it’s essential to choose an EQMS that you can configure to your processes.
It’s not uncommon for food and beverage companies to look for software that comes with static, pre-configured processes. The problem with this approach is that it inevitably means adjusting your processes to fit the software. The result is less efficiency from the outset as well as future roadblocks to continuous improvement.
Instead, you should look for solutions that configure to your existing processes, including:
- Document management
- Employee training
- Corrective action
- Audit management
- Supplier quality management
- Non-conformance tracking
- Complaint management
- Change management
In addition to being able to configure processes, the system you choose must support FDA and USDA compliance. Specifically, the software should provide capabilities including 21 CFR Part 11 electronic signatures and audit trails to streamline regulatory reporting.
Finally, you’ll want to make sure your food and beverage quality management system is mobile-ready, promoting successful user adoption by giving operators the freedom to move around between high-speed lines.
Define Your Food and Beverage Quality Management Processes
Before you roll out any automated processes, you need to take the time to review, define, and optimize those processes. Once you have that, you’ll be in a good position to determine exactly how you will need to configure each process solution.
Documents should incorporate requirements from areas such as:
- Regulatory requirements
- Customer requirements and contract items
- Certification standards such as Safe Quality Food (SQF)
- Sanitizing and preventive maintenance
- Specification labeling
- Packaging
- Food safety plan
- Hazard Analysis and Critical Control Points (HACCP) procedures
- Foreign Supplier Verification Program (FSVP) required under the FDA Food Safety Modernization Act (FSMA)
For additional information on defining your quality processes, refer to our blog, A Successful QMS Implementation Requires Clearly Defined Processes.
Deploy Your Document Control System
Companies take different approaches to how they deploy different EQMS solutions, often starting with corrective actions in response to audit findings or complaints. As a best practice, however, we recommend starting with your document control system and then following with employee training.
Documents are the foundation of your entire food safety management system, including your food safety plan, HACCP plan, FSVP, sanitation and pest control procedures, and more. All of these are top FDA citations, and without a document control system you leave yourself open to audit findings, 483 inspectional observations, and warning letters.
Deploying other solutions before document control is like putting up a housing development without sewers, water, electricity and roads. In the context of the Plan-Do-Check-Act approach, it’s the first step in the planning process, where you say what you are going to do. Only then can you go back and verify whether you are doing what you say.
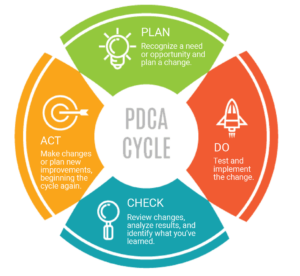
Plan-Do-Check-Act (PDCA) Cycle
Deploy Your Employee Training Management System
After deploying your document control system, the next step is rolling out employee training management. This approach means that once you standardize your documents, you can ensure people have the knowledge and skills to execute those processes.
Furthermore, any time a document or process is changed, that will also require updating employee training requirements in the EQMS. This includes when you deploy additional processes within your food and beverage compliance management system.
Digital data from document control and training solutions lets you define metrics to track and trend system performance, providing detection and alerts to escalate potential gaps like late or missed training.
Deploy Your Supplier Quality Management System
Given today’s supply chain challenges and the fact that supplier quality is a major source of variation, manufacturers should prioritize rolling out their supplier quality management solution.
Best practices include:
- Using supplier scorecards to benchmark suppliers and assess risk
- Tracking supplier non-conformances and corrective actions
- Assigning action items to suppliers in the EQMS to streamline communication and ensure timely follow-up on quality issues
Integrate Additional Quality and Compliance Processes
As you roll out additional capabilities in your EQMS such as corrective action, supplier quality management and audit management, you’ll want to link each one together into streamlined workflows.
This is easier to do if you have a configurable EQMS to start with. For example, when an issue comes up with a supplier, you may need to:
- Initiate a corrective action and assign action items within the EQMS directly to the supplier and others
- Add new incoming inspection requirements
- Add questions to be used during external audits
- Update the supplier scorecard to reflect the issue’s impact on the supplier’s overall risk rating
In addition to connecting EQMS processes, you can link quality data with other systems such as finance, CRM, supplier management, and business intelligence reporting tools. Being able to push and pull data from one system of record to another is essential to efficiency, data quality, and data integrity, while making it easier to identify trends and risks.
AssurX pre-configured workflows can be modified for any process to meet unique organizational needs with easy to use drag-and-drop functionality.
Conclusion
Automating your food and beverage quality and compliance management is a significant undertaking, but it’s well worth the effort. Start with a configurable EQMS, and then define your processes and link them together into integrated workflows. In the end, the result is a safer, more agile food safety management system. An integrated system that meets FDA and USDA requirements provides a strategic investment for your business while protecting consumers.



